Milvue Secures Its Second FDA Clearance, Strengthening Its Leadership in Medical AI
Montreal, 02/03/2025 – Milvue obtains FDA clearance for TechCare Trauma, its AI-powered X-Ray solution for musculoskeletal pathology detection and triage. This milestone makes Milvue the first company to offer a fully integrated fracture detection suite across the US, Canada, and Europe, and the only provider of a combined “bone and lung” AI solution in the US, with TechCare Trauma complementing SmartChest, its triage tool for pulmonary pathologies.
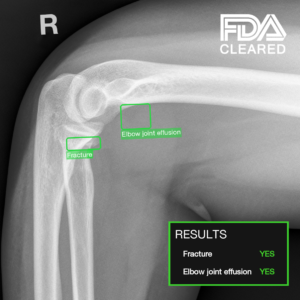 TechCare Trauma: AI-Powered X-Ray Detection of Fractures and Elbow Joint Effusions, Now in the US.
TechCare Trauma: AI-Powered X-Ray Detection of Fractures and Elbow Joint Effusions, Now in the US.
Powered by AI, TechCare Trauma enables real-time detection and localization of fractures and elbow joint effusions.
Already ranked #1 in Europe for fracture detection, this solution is now available in the US market either directly or through a network of partners.
A Comprehensive Solution for Adults and Pediatrics.
Designed for both adult and pediatric radiology, TechCare Trauma provides invaluable support for detecting elbow trauma in children (ages 2 and up), particularly through the analysis of joint effusions.
TechCare Trauma detects fractures with no age restrictions.
TechCare Trauma: Proven Accuracy and Enhanced Diagnostic Performance Across Diverse Clinical Settings
Validated on a cohort of over 7,000 U.S. patients, TechCare Trauma demonstrated high accuracy and consistent performance across different anatomical regions, imaging views, patient demographics (age and sex), imaging hardware manufacturers, and both displaced and non-displaced fractures.
In a study involving 24 experienced physicians with different levels of expertise in MSK, radiology, and pediatric care, TechCare Trauma improved diagnostic sensitivity by 20% for fractures and 18% for elbow joint effusions, while also significantly reducing interpretation time, enhancing security and productivity in clinical workflows.
Seamlessly integrated into healthcare workflows, TechCare Trauma is available via a secure cloud-based platform or on-premises deployment.
Milvue: A Global AI Powerhouse with Rapid Growth
Founded in 2018 in France, Milvue has quickly established itself as a global leader in AI-powered medical imaging. Operating across five geographical areas (Europe, North America, Latin America, the Middle East, and Africa), the company analyzed over 25 M X-rays in 2024 for hospitals and imaging centers worldwide.
Its AI solutions support the entire diagnostic workflow for radiologists, emergency physicians, and orthopedic specialists, offering advanced triage, detection, and measurement tools.
After obtaining CE IIa certification in 2020 and Health Canada license in 2023, Milvue now achieves a major milestone with its second FDA clearance, reinforcing its commitment to innovation and patient safety.
Learn more about TechCare Trauma: https://www.milvue.com/us/solutions/techcare-trauma/
FDA clearance. TechCare Trauma is a class II medical device according to US regulations. Please consult IFU (Instructions For Use) of the product for more information (Rx only).
Press Contacts
📩 Hortense Bachelier – VP Marketing & Communication
📧 hortense@milvue.com
📩 Aïssa Khelifa – CEO, Milvue North America
📧 aissa@milvue.com | 📞 +1 (438) 459 29 58
Upload the Press Release:
from Milvue
